
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Endocrinol., 01 March 2019
Sec. Cellular Endocrinology
Volume 10 - 2019 | https://doi.org/10.3389/fendo.2019.00087
This article is part of the Research TopicMelatonin in Health and DiseaseView all 9 articles
The pathophysiological function of the G-protein coupled melatonin MT1 and MT2 receptors has not yet been well-clarified. Recent advancements using selective MT1/ MT2 receptor ligands and MT1/MT2 receptor knockout mice have suggested that the activation of the MT1 receptors are mainly implicated in the regulation of rapid eye movement (REM) sleep, whereas the MT2 receptors selectively increase non-REM (NREM) sleep. Studies in mutant mice show that MT1 knockout mice have an increase in NREM sleep and a decrease in REM sleep, while MT2 knockout mice a decrease in NREM sleep. The localization of MT1 receptors is also distinct from MT2 receptors; for example, MT2 receptors are located in the reticular thalamus (NREM area), while the MT1 receptors in the Locus Coeruleus and lateral hypothalamus (REM areas). Altogether, these findings suggest that these two receptors not only have a very specialized function in sleep, but that they may also modulate opposing effects. These data also suggest that mixed MT1-MT2 receptors ligands are not clinically recommended given their opposite roles in physiological functions, confirmed by the modest effects of melatonin or MT1/MT2 non-selective agonists when used in both preclinical and clinical studies as hypnotic drugs. In sum, MT1 and MT2 receptors have specific roles in the modulation of sleep, and consequently, selective ligands with agonist, antagonist, or partial agonist properties could have therapeutic potential for sleep; while the MT2 agonists or partial agonists might be indicated for NREM-related sleep and/or anxiety disorders, the MT1 agonists or partial agonists might be so for REM-related sleep disorders. Furthermore, MT1 but not MT2 receptors seem involved in the regulation of the circadian rhythm. Future research will help further develop MT1 and/or MT2 receptors as targets for neuropsychopharmacology drug development.
Following Tononi and Cirelli's synaptic homeostasis hypothesis (1), sleep is the price the brain pays for plasticity. Indeed, during waking, the learning process requires the strengthening of connections throughout the brain. This process increases cellular need for energy and supplies, decreases signal-to-noise ratios, and saturates learning. During sleep, cerebral spontaneous activity renormalizes the net synaptic strength and restores cellular homeostasis. This activity of synapses during sleep may also explain the benefits of sleep on memory acquisition, consolidation, and integration (1).
In mammals, physiological sleep is comprised of two distinct states called rapid-eye movement (REM) sleep and non-REM (NREM) sleep that alternate through the night in a cyclical fashion. REM occurs in short periods, characterized by a decrease in muscle tone and associated with a profound sympathetic activation, including increased heart rate, breathing, blood pressure, and temperature. NREM periods are longer and are associated with a parasympathetic activation, consisting of low blood pressure, low heart rate, and decreased temperature. While structured dreams occur mostly in REM, non-structured and bizarre dreams occur in NREM. In adults, about 75–80 percent of total time spent in sleep is spent in NREM sleep while the remaining 20–25 percent occurs in REM sleep. During the night, adult subjects usually experience four to five NREM to REM sleep cycles. Interestingly, newborns spend more time in REM, and the time spent in NREM increases progressively over the years at the expense of REM.
NREM sleep is divided into progressively deeper stages—named stage N1, stage N2, and stage N3—that can be distinguished based on specific electroencephalogram (EEG) traits [for details on this topic, which is beyond the aim of this review, please see Atkin et al. (2) and Iber et al. (3)]. However, it is important to highlight that stage N3, commonly referred to as slow wave sleep (SWS) during which there is deep or delta-wave sleep, seems important for cerebral restoration and recovery, the maintenance and consolidation of memory (4), and metabolic regulation (5). As a consequence, disturbances in the duration and architecture of sleep is often associated with next-day impairments in conducting daily activities and, if not treated, can be closely linked to many neurological and psychiatric disorders (6–8). The lack or the disruption of sleep, known as “insomnia,” is a common public health problem, with a prevalence ranging from 11 to 16% (9).
The publication of the 5th edition of the Diagnostic and statistical manual of mental disorders (DSM-V) (10) fundamentally changed the landscape of sleep medicine and the diagnosis of insomnia. The DSM-IV distinguished primary insomnia [characterized by a difficulty to initiate or maintain sleep for at least 1 month, with associated daytime fatigue, significant distress or social impairment (9, 10)] from insomnia secondary to another diagnosis (including major depressive disorder and generalized anxiety disorder). Instead, the DSM-V has eliminated primary insomnia as a diagnosis in favor of “insomnia disorder,” which may occur alongside other diagnoses like major depressive disorder. This revised definition obliges the clinician to treat insomnia as a distinct mental condition, even if it may be present with other mental disorders (2).
Insomnia is frequent in people suffering from major depression, with alterations in sleep neurophysiology, notably decreased SWS, reduced REM latency and increased REM density. Increased REM density has also been observed in eating disorders, narcolepsy, presenile dementia, and other neuropsychiatric diseases (11).
Besides “insomnia disorder,” mostly characterized by a decrease in NREM quantity and longer latency to sleep (first episode of NREM), the DSM-V, like DSM-IV, proposes a specific classification for REM sleep behavior disorders. REM sleep behavior disorders are characterized by recurrent episodes of arousal during sleep associated with vocalization and/or complex motor behaviors that arise during rapid eye movement (REM) sleep, confusion or disorientation on waking from these episodes, co-presence of REM sleep without atonia on polysomnographic recordings, and/or history of synucleinopathy diagnosis (e.g., Parkinson's disease, multiple system atrophy).
From a pharmacological point of view, it is thus important that a drug used to treat insomnia or sleep-related disorders not only acts on the duration of sleep but also preserves the physiological sleep architecture. Unfortunately, most of the currently available hypnotics considerably alter the physiological sleep architecture (2). In addition, official medicine has not yet recognized guidelines for specific treatment of “NREM disorders” vs. “REM disorders,” and hypnotics are non-differentially used for both conditions.
Currently used hypnotic drugs, such as benzodiazepines and thier derivates (i.e., zopiclone), act mostly on the GABAergic system, increasing SWS and decreasing REM sleep, thus altering the sleep architecture (2). This can result in next-day cognitive impairments and may also lead to abuse. Antidepressants, such as tricyclics and selective serotonin reuptake inhibitors (SSRIs) mostly reduce REM density, with little or no effect on SWS. The catecholamine releaser bupropion increases REM and has no effect on SWS (2, 12, 13). To develop new effective hypnotic drugs that selectively increase SWS without altering REM density and the whole sleep architecture therefore remains a scientific and medical challenge.
The physiological effects of melatonin (N-acetyl-5-methoxytryptamine, MLT) in the brain result from the activation of high-affinity (Ki ≈ 0.1 nM), G protein-coupled receptors, referred to as MT1 and MT2. Activation of both receptors mainly activates Gi proteins with inhibition of adenylyl cyclase and subsequent decrease of intracellular cAMP levels. Detailed information on the molecular signaling pathways activated by melatonin receptors is beyond the scope of the aim of the present work and can be found in the reviews by Dubocovich et al. (14), Jockers et al. (15), and Oishi et al. (16). However, of interest, recent lines of research have indicated that melatonin receptors can form abundant MT1/MT2 hetero-oligomers and that they can both heteromerize with other receptors, including the serotonin 5-HT2C (17). Importantly, from both neurobiological and pharmacological perspectives, these heteromers display functional properties different from those of the corresponding homomers (17). For example, in the MT2/5-HT2C heteromer, melatonin binding induces the activation of Gq signaling through a transactivation of the serotonergic receptor caused by conformational changes of the MT2, which is normally not coupled to a Gq (17).
Due to the lack of selective ligands for MT1 and MT2 receptors, the respective roles of these receptors in brain function and in particular in sleep regulation remain unclear.
The neuromodulator MLT is synthesized by the pineal gland and has been reported to have hypnotic effects on humans, although these results are still controversial (18–21). Meta-analysis on the effects of melatonin indeed suggest that melatonin has a soporific effect, helping people to fall asleep, but has no effects on sleep maintenance and sleep quality (18, 19).
Similarly, in laboratory animals, several studies have demonstrated that MLT reduces time to sleep onset and increases SWS and REM (22–24), effects that would be blocked by the GABAA receptor antagonists flumazenil and picrotoxin (24). Others have suggested that MLT regulates REM, since lesioning of the pineal gland or the inhibition of MLT synthesis reduce REM density during light and dark periods (25–27). The effects of MLT (3–5 mg/kg) in Djungarian hamsters and rats (both nocturnal animals) were short lasting and depended on the time of day. MTL prolonged sleep latency in the late light period, enhanced sleep fragmentation in the early light period, and elevated body temperature. REM sleep was reduced when hamsters were treated with MLT after the late light period and when rats were treated after dark onset. These indicate that MLT induces changes that are typical for the dark period of each species, i.e., wakefulness in the nocturnal Djungarian hamster and rat, and sleepiness in diurnal animals (28).
Electrophysiological recordings in monkeys have indicated that MLT has only a weak and transient effect on sleep in these species (29, 30), decreasing the latency of the first episode of sleep (31).
Five non-selective MT1/MT2 agonists—ramelteon (S)-N-[2-(1,6,7,8-tetrahydro-2H-indeno[5,4-b]furan-8-yl)ethyl]propionamide, tasimelteon (VEC-162; structure not disclosed), TIK-301 (β-methyl-6-chloroMLT; N-[(2R)-2-(6-chloro-5-methoxy-1H-indol-3-yl)propyl]acetamide), agomelatine (N-[2-(7-methoxynaphthalen-1-yl)ethyl]acetamide) and piromelatine (N-[2-(5-methoxy-1H-indol-3-yl)ethyl]-4-oxopyran-2-carboxamide—have been tested in different species for potential use in insomnia. Ramelteon seems to have insignificant effects on sleep in rats (32), monkeys (31), and cats (29). Agomelatine, on top of being a non-selective MT1/MT2 agonist, also acts as an antagonist at the level of 5-HT2C receptors. Agomelatine increases NREM and REM sleep in rats but only if administered shortly before the dark phase (active phase for rodents), but not during the light phase (inactive phase for rodents) (32). In the same experiment, melatonin increased REM sleep, which was followed by an increase in wakefulness (32). Tobler et al. (30) found that melatonin and agomelatine (S-20098) reduced the power density in NREM sleep in the low frequency range (1–8 Hz), but did not affect the vigilance states and brain temperature. Sleep data with tasimelteon and TIK-301 in rats are lacking (33).
A summary with the preclinical data investigating the effects of melatonin and non-selective MT1/MT2 agonists on the sleep/wake cycle of rats is reported in Table 1.
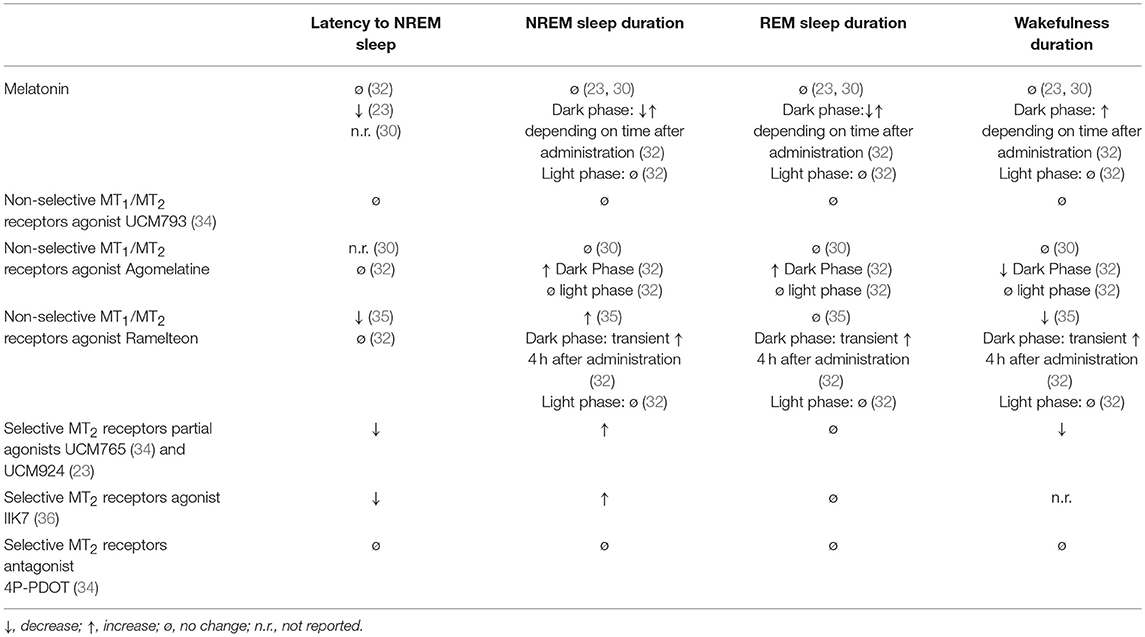
Table 1. Acute effects of melatonin, non-selective MT1/MT2 receptors agonists, and selective MT2 receptors partial agonists, agonists and antagonists on sleep/wake stages of rats during the 24-h light/dark cycle.
Ramelteon (37–39), tasimelteon (40), and TIK (41, 42) have also been tested in humans for the treatment of insomnia. All three significantly reduced the latency to sleep in humans, but their effect on total sleep time was minimal.
In particular, the non-selective MT1-MT2 receptor ramelteon decreases the latency of sleep but not the whole duration (39, 43) and for this reason was approved by the Food and Drug Administration (FDA, United States) but not the European Medicines Evaluation Agency (EMEA) because “.. the difference in the time taken to fall asleep between patients taking Ramelteon and those taking placebo was considered to be too small.…When other aspects of sleep were considered, Ramelteon did not have any effect.” (https://www.ema.europa.eu/medicines/human/withdrawn-applications/ramelteon, consulted on November 1, 2018).
Similarly, the non-selective agonist tasimelteon (VEC-162) was effective for treatment of transient insomnia associated with shifted sleep and wake time (44) and was developed as an orphan drug for the treatment of Non-24-H Sleep-Wake Disorder, but not for insomnia. The EMEA approved tasimelteon for the same condition but only in completely blind people (https://www.ema.europa.eu/documents/assessment-report/hetlioz-epar-public-assessment-report_en.pdf, consulted on November 1, 2018).
Agomelatine was also approved by the EMEA as an antidepressant, but a recent meta-analysis has pointed out its low effects compared to other classes of antidepressants (45), some clinical evidence has shown that agomelatine could be efficacious in sleep disorder (46), especially if associated with depression (47).
Piromelatine is a MT1and MT2 agonist with agonism also at 5-HT1A/1D receptors (48). Piromelatine was shown to have both hypnotic and antinociceptive effects by electroencephalogram (EEG) recordings in an animal model of neuropathic pain, partial sciatic nerve ligation (PSL) (49). It increases NREM sleep and decreases wakefulness in PSL mice, but the effect could be blocked by preadministration of a melatonin receptor antagonist, a 5-HT1A receptor antagonist, or an opiate receptor antagonist (49), demonstrating a lack of selectivity for the melatonin receptors.
In 2013, Neurim Pharmaceuticals Ltd announced positive results from a phase II randomized clinical trial (N = 120) of piromelatine for the treatment of primary insomnia (50). Active treatment with piromelatine at 20 or 50 mg/d over 4 weeks resulted in significantly improved wake after sleep onset (WASO). However, the primary outcome of latency to persistent sleep was not significant when compared with the placebo (https://clinicaltrials.gov/ct2/show/results/NCT01489969) and consequentiality, the company did not further develop piromelatine for insomnia. The Clinicaltrials.gov database lists a study currently recruiting patients entitled “Safety and Efficacy of Piromelatine in Mild Alzheimer's Disease Patients (ReCOGNITION),” https://clinicaltrials.gov/ct2/show/NCT02615002, indicating that the compound will be primarily developed for cognition and not for sleep (a secondary outcome of the study).
Altogether, these animal and clinical studies have pointed out the equivocal effects of non-selective agonists on sleep duration, despite the undoubted evidence that MLT receptors are implicated in sleep regulation and circadian rhythms.
Sleep is regulated by two processes, the sleep/wake homeostasis and the circadian clock (51). In mammals, the master circadian clock is located in the suprachiasmatic nucleus (SCN) of the hypothalamus. The SCN receives direct inputs about the external environmental day/night cycle from the retina via the retino-hypothalamic tract, and then accordingly, controls the synthesis of melatonin by the pineal gland. In turn, melatonin controls the SCN activity via a feedback mechanism involving MT1 and MT2 receptors located in the SCN (52).
In our lab, we used electroencephalogram (EEG) and electromyogram (EMG) recordings in rats for 24 h to examine the effects of the selective MT2 partial agonists UCM765 and UCM924 on sleep in comparison with diazepam, melatonin and the non-selective MT1-MT2 agonist UCM793 (23, 34).
We observed that UCM765 decreased the latency to the first episode of NREM sleep and increased the total amount of NREM sleep, in particular during the light (non-active) phase. We then compared the effects of UCM765 with those of the clinically-used hypnotic drug diazepam, and observed similar effects on both latency to the first episode and duration of NREM sleep. However, we found that, unlike diazepam, UCM765 did not induce a significant suppression of delta power activity during NREM sleep (34). Similar to melatonin, the MT1/MT2 non-selective agonist UCM793 did not produce significant effects on sleep stages (34), suggesting that the MT2 receptor subtype is probably the one mainly involved in the regulation of NREM sleep but the MT1 may counterbalance the MT2-mediated effects. This hypothesis is also supported by the fact that knockout mice for both MT1 and MT2 receptors (53), as well as pinealectomized rats (54), do not show impairments of NREM and REM sleep duration. However, we cannot exclude the possibility that melatonin acts on sleep through mechanisms independent of MT1/MT2 activation. Indeed, unlike the non-selective MT1/MT2 agonist UCM793 (34), melatonin significantly reduced the latency to NREM sleep onset but not to REM sleep onset (23). Evidence has shown that melatonin can interact with other neurotransmitter systems implicated in the neurobiology of sleep (2) including alpha-7 nicotinic (55, 56) or GABA (24) receptors and the release of serotonin (57, 58).
Targeting the MT2 receptors with the MT2 agonist IIK7 also selectively increased the duration of NREM sleep without affecting REM sleep, although the overall effects appear to be only transient (36). Future studies should investigate possible differential effects on NREM sleep produced by partial and full agonists toward the MT2 receptors. There is not yet a clear understanding of whether and how MT1 and MT2 receptors may desensitize upon stimulation by exogenous melatonin/selective ligands and according to the daily fluctuating levels of endogenous melatonin. Nonetheless, it appears that the hypnotic effects of MT2 partial agonists may be superior to those of MT2 full agonists, because the former would avoid the rapid desensitization induced by the full agonist. The higher pharmacological efficacy of MT2 partial agonists over melatonin has also been found when comparing their analgesic effects in preclinical models of neuropathic pain (59, 60).
Given the paucity of selective MT1 receptor ligands, only one pharmacological study has explored the effects of MT1 receptor activation/inhibition upon the sleep stages, indicating a possible selective effect of MT1 receptor selective ligands on REM sleep activation (61).
UCM765 and UCM924 have shown high selectivity and affinity toward MT2 receptors and low affinity toward a panel of many other receptors (59) known to be involved in sleep (2, 62). Furthermore, the role of MT2 receptors in the observed effects of these two drugs on sleep has been validated using both pharmacological and genetic approaches. The MT2 receptor antagonist cis-4-phenyl-2-propionamidotetralin (4P-PDOT) is a reference compound exhibiting good binding affinity for the human cloned MT2 receptor (pKi = 8.8), and a selectivity for the MT2 receptor at least 100 fold that of the MT1 subtype (63). In order to test the hypothesis that the promotion of NREM sleep is MT2-mediated, we administered 4P-PDOT (10 mg/kg, a dose not affecting sleep stages) 10 min prior to UCM765, and found that 4P-PDOT completely blocked the effects of UCM765 on NREM sleep duration (34). UCM765 was also tested in MT2KO, and, unlike in wild-type control mice, the compound did not enhance NREM sleep in the MT2KO animals. These data strongly confirm the important role of MT2 receptors in modulating NREM sleep.
The role of melatonin receptors in sleep has also been investigated by taking advantage of knockout mice for MT1 and/or MT2 receptors.
Quite surprisingly, the lack of both MT1 and MT2 receptors did not significantly affect the amount of NREM and REM sleep during the 24 h (53). In contrast, a slight but significant increase in the time of wakefulness during the 24 h was present (53). These findings suggest that the lack of both melatonin receptors only minimally influences the two sleep stages, in agreement with the finding that also the lack of melatonin (their physiological ligand) due to a pinealectomy does not significantly affect the duration of sleep (54).
In keeping with the pharmacological studies reported above, the genetic inactivation of only one of the two melatonin receptor subtypes instead produces significant effects on the sleep stages. MT2KO mice display a significant reduction of NREM sleep duration during 24 h, with the decrease mainly due to an effect occurring during the light (inactive) phase (34, 53). No effects on REM sleep duration have been observed in MT2KO mice (34, 53). These findings in MT2KO mice corroborate pharmacological findings with MT2 agonists/partial agonists (23, 34, 36) demonstrating a selective role of MT2 receptors in regulating NREM sleep.
In MT1KO mice a significant decrease in the duration of REM sleep has been observed (34, 53), suggesting a central role for MT1 receptors in REM sleep regulation. In contrast, the possible involvement of MT1 receptors in NREM sleep remains unclear. While in rats there is concordance among studies on how to score sleep stages, different protocols have been used in mice. In particular, sleep is scored using either 4 or 10 s epochs (64). However, given that in mice very short episodes of REM sleep are present, the 4 s epoch seems probably the best way to score sleep in mice (64). In keeping with this rationale, we found that the duration of NREM and REM sleep in MT1 mice can slightly differ depending on the 4 or 10 s methodological approach. Using 4 s epochs, we found no change in NREMS in MT1KO mice compared with WT control animals. In contrast, using 10 s epochs we found a slight but significant increase of NREM sleep during the dark/active phase in MT1KO mice compared with WT. These different results with 4 and 10 s analyses suggest a disruption of microarchitecture of REM in MT1KO; moreover, the opposing effects in NREM detectable with the 10 s analyses point out the opposing effects of MT1 and MT2 on NREM sleep: while MT1KO have an increase in NREM, the MT2KO have a decrease.
Interestingly, MT1KO also show an impairment at the level of dark-light cycle of the REM sleep: the quantity of REM is the same in the dark and light periods, suggesting the involvement of this receptor also in the circadian regulation of REM sleep.
Collectively, as summarized in Table 2, the study of the 24-h sleep/wake cycle in melatonin receptors knockout mice indicates that MT1 receptors are mostly involved in REM sleep regulation while MT2 receptors in NREM sleep.
The SCN is the pacemaker of the circadian rhythms in the body, including the sleep-wake cycle. Both MT1 and MT2 receptors have been reported at the level of the SCN; however, while the presence of MT1 receptors has been demonstrated with several techniques such as RT-PCR, in-situ hybridization and immunohistochemistry (65–67), the data on the presence of MT2 receptors are not yet so clear and points only to a very low expression (65, 67, 68). Our laboratory has shown that MT2 receptors are located in critical areas for sleep functions. From rostral to caudal, strong, selective MT2 immunoreactivity of neuronal cell bodies and proximal dendrites was consistently observed in key brain regions: the septum, CA2 layers of the hippocampus, supraoptic nucleus, reticular nucleus of the thalamus, red nucleus, substantia nigra pars reticulata, oculomotor nuclei, and ventral tegmental nucleus (65). Moderate MT2 immunoreactivity was also seen in the ventral pallidum, internal globus pallidus, other sectors of the hippocampus (e.g., the dentate gyrus), paraventricular nucleus of the hypothalamus and inferior colliculus (65).
The reticular thalamus (RT) is a small area whose activation promotes NREM sleep by connecting deeper brain structures to cortex via thalamo-cortical pathways. RT generates the classic silent/burst rhythmic activity during episodes of NREM sleep (69–71). During episodes of NREM sleep, RT neurons discharge in a slow, rhythmic, burst-firing mode that is transmitted to thalamic relay nuclei and modulated by corticothalamic inputs, resulting in a widespread synchronization across neuronal assemblies (72, 73). In rats, the selective MT2 receptor partial agonist UCM765 induces at the level of RT neurons a rhythmic synchronized burst activity separated by periods of silence, characterized by an increased percentage of spikes in burst, an increase in mean spike per burst and a decrease in mean interburst time (34). Since this rhythmic activity promotes NREM sleep, MT2 receptors may thus be viewed as a key component in sleep regulation. Of note, the activation of RT neurons by UCM765 is MT2 receptor-mediated, since the local infusion of 4P-PDOT blocked the effects of the drug upon the neurons, and is sufficient to promote NREM sleep. Indeed, when UCM765 is injected in a brain region not primarily involved in sleep regulation but containing MT2 receptors such as the substantia nigra pars reticulate, no effects on NREM sleep has been observed (34).
Recently, Sharma et al. (74) found in mice that orexin neurons in the perifornical lateral hypothalamus (PFH) express MT1 but not MT2 receptors. Orexins, also known as hypocretins, are neuropeptides synthesized in the brain exclusively by neurons in the lateral hypothalamic area that makes excitatory connections to all of the arousal-promoting nuclei. Orexins are thus a crucial neurotransmitter in promoting wakefulness, and indeed melatonin injected at the level of PFH was able to induce sleep (74). Following this finding, Sharma et al. (74) claimed that melatonin via MT1 receptors in the PFH may induce sleep. It is our opinion that this claim requires further proof-of-concept studies (75), but MT1 receptors present in the PFH are likely to contribute to effects of melatonin upon the sleep-wake cycle.
We also found MT1 receptors at the level of 5-HT neurons in the dorsal raphe (65), and the lack of MT1 receptors in MT1KO mice impaired the physiological light-dark fluctuation of a subpopulation of dorsal raphe 5-HT neurons (76). Monoaminergic neurons fire at a steady rate during wakefulness, decrease their firing during NREM sleep, and are virtually silent during REM sleep (2). Future studies are thus warranted to examine whether MT1 receptors present on 5-HT neurons are involved in the modulation of sleep.
It is important at this point to improve our understanding of how the MT1 and MT2 receptors play their roles in the complex sleep circuitry composed of different brain nuclei and receptors.
The neural circuits that generate arousal and sleep (both NREM and REM) remain to be completely elucidated.
Humans are diurnal mammals, with a circadian clock that promotes wakefulness during the day. Sleep timing is phase-linked to intrinsic circadian rhythm-controlled temperature rhythms as well as extrinsic light and dark signaling (77). Homeostasis is another sleep regulator, meaning that the decrease of sleep for one night induces an increase in deep sleep quantity and quality the following night.
The manner in which the brain alternates cycles of NREM and REM remains unknown; however, a prominent role for melatonin receptors can be hypothesized. The melatonin receptors MT1 and MT2 are both present at the level of retina, but MT2 mRNA seems to be absent in retinal ganglion cells (78). The retinohypothalamic tract, which contains the intrinsically photosensitive retinal ganglion cells (ipRGC) and the photopigment melanopsin, inputs directly and monosynaptically to the SCN, an area rich in MT1 and MT2. Circadian signals from the SCN are transmitted sequentially to the paraventricular nuclei (PVN), intermediolateral nucleus of the spinal cord (IML), superior cervical ganglion (SCG), and finally the pineal gland (79). Bilateral SCN lesion abolishes circadian rhythms of melatonin synthesis and secretion, demonstrating that the SCN is the melatonin rhythm generator (80). The pineal gland produces melatonin when stimulated by the SCN glutamatergic neurons (in response to the darkness) (79). MLT is then released into the bloodstream through which it reaches every organ in the body, including the brain where it interacts with MT1 and MT2 located in the NREM areas (including RT) or REM area [including locus coeruleus (LC) and lateral hypothalamus (LH)]. These areas regulate in concert the different sleep cycling. It may be hypothesized that the peak of melatonin between 12 and 3 a.m. may desensitize or down-regulate its own receptors, generating a differential expression and/or sensitivity of MT1 (REM sleep) and MT2 (NREM sleep) that may in their turn generate a kind of rhythmic balance between NREM, REM and wakefulness. In support of this theory, it has been shown that MT2 receptors desensitize quickly after melatonin exposure (81).
Melatonin stimulates the brain's MT2 receptors in the NREM sleep-activating regions of the brain: the reticular thalamus and the preoptic areas, including both the ventrolateral preoptic area (vlPO) and the median preoptic area (MNPO) (34, 65). Specifically, the MNPO appears to regulate the firing activity of the vlPO (82). During the transition from wakefulness to sleep, the MNPO—which specifically contains neurons that fire during SWS and paradoxical or REM sleep, with slow discharging activity <5 Hz—begin to fire not before, but after, sleep onset, with a gradual increase in discharge rate (83).
During NREM sleep, two nuclei are particularly active: the RT, containing melatonin MT2 and GABA receptors and responsible for thalamocortical input to the prefrontal cortex (showing synchronized activity during NREM); and the ventrolateral preoptic area (vlPAG), containing GABA and galanin receptors, and inhibiting noradrenergic, serotonergic, cholinergic, histaminergic, and hypocretinergic neurons. These nuclei play a role in the “reciprocal inhibitory” model of the sleep–wake switch. In particular, during NREM sleep, the vlPO sends inputs that reduce the activity of the orexinergic arousal system and the monoamine nuclei [including the Ventral tegmental area (VTA) containing dopamine (DA) neurons, the dorsal raphe (DR) containing serotonin (5-HT) neurons, and the LC containing norepinephrine (NE) neurons] by releasing the inhibitory neurotransmitters GABA and galanin. As a feedback mechanism, vlPO neurons receive reciprocal inputs from the arousal nuclei including the VTA, DR, and LC; the vlPO also receives input from the histaminergic tuberomammillary nucleus (TMN) (84).
People suffering from fatal familial insomnia (FFI) show thalamic disruption that inactivates their ability to sleep, which is paralleled by a dysfunction in melatonin production (85). As mentioned before, the RT neurons discharge in burst activity exclusively during NREM, and thalamocortical pathways project this synchronous burst activity, intermingled with periods of silence, onto the cortex. This rhythmic firing activity generates the synchronized EEG pattern typical of SWS, which produces disconnection between the cortex and the outside world (86). Remarkably, the RT is also rich in melatonin MT2 receptors, which are likely activated at the beginning of NREM sleep (34). These receptors, which are contribute to the generation of the characteristic bursts that, through the thalamo-cortical pathways, produce the classical silent/burst activity in the PFC. Conversely, during wakefulness, the RT and thalamocortical neurons are depolarized by inputs from the reticular activating system of the brainstem, and discharge instead with a tonic activity [adapted from Purves et al. (87)].
On the other hand, REM sleep is regulated by other brain areas. The vlPAG is a putative “REM ON” nucleus, switching the brain to the REM sleep mode. During REM, the sublateral nucleus (SLD), the basal forebrain (BF), and the lateral tegmentum/ pedunculopontine tegmentum (LDT/PPT, rich in acetylcholine receptors) and the ventromedial medulla (VM) neurons become particularly active.
Many researchers have hypothesized that REM sleep is mediated mostly through cholinergic neurons located in the LDT/PPT. These neurons are active during REM sleep and generate the cortical activation and atonia typical of this sleep stage, and are inactive during NREM sleep. Indeed, LDT/PPT neurons send inputs to the ventromedial medulla (VM), which inhibits motor neurons by releasing GABA and glycine into the spinal and brainstem motor neurons, producing atonia. LDT/PPT neurons are also the main source of acetylcholine (Ach) to the thalamus: activation of this ACh pathway depolarizes thalamic neurons, generating the cortical activation associated with REM sleep and dreaming. Other nuclei important for REM sleep regulation are: (1) the sublaterodorsal nucleus (SDL) which produces GABA and glutamate and projects to the glycinergic/GABAergic premotor neurons in the ventromedial medulla and ventral horn of the spinal cord, and through these circuits likely inhibits motor neurons during REM sleep; (2) the melanin-concentrating hormone (MCH)-containing neurons that fire during REM sleep and decrease their activity during NREM sleep and wakefulness [ Saper et al. (88); reviewed in España and Scammell (62)]; and (3) LC neurons that fire as a function of vigilance and arousal displaying a firing of 4–6 Hz during quiet wakefulness and a sustained activation during alertness or stress. LC NE firing decreases markedly during NREM and is completely silent during REM sleep (89, 90).
Interestingly, we found that the daily circadian changes of LC NE neural activity are blunted in MT1KO mice as compared with WT controls, and the bust-firing activity of LC NE neurons, that is associated with the synaptic release of the neurotransmitter (91), is significantly reduced in MT1KO compared with WT mice (76).
Another cholinergic nuclei that is active during REM sleep and wakefulness is the LH which contains both MT1 and orexin receptors (74).
However, more research, especially with selective compounds or optogenetic techniques, is required to better differentiate the role of these two receptors in sleep regulation. Figure 1 illustrates the main areas of the brain implicated in the regulation of sleep and wakefulness with their respective receptors, including MT1 and MT2.
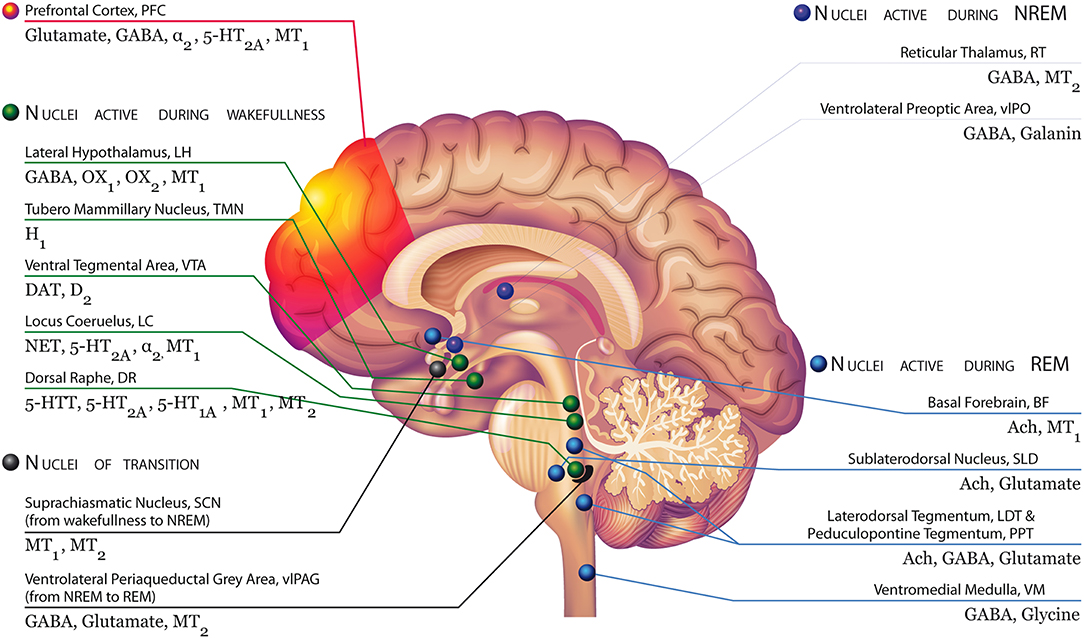
Figure 1. Brain areas involved in the regulation of sleep and wakefulness with their respective receptors, including MT1 and MT2 receptors Modified with permission from Atkin et al. (2). Top left, green: During NREM, the serotonin neurons of the Dorsal Raphe (DR), the dopaminergic neurons of the Ventral tegmental area (VTA), and the noradrenergic neurons of the Locus Coeruleus (LC) decrease their firing activity. These neurons are silent during REM. OX1 and OX2-containing orexinergic neurons of the Lateral Hypothalamus (LH) decrease their firing activity during NREM and REM. The histaminergic H1-containing neurons of the Tuberomammillary Nucleus (TMN) decrease their firing activity during sleep. During wakefulness, neurons of the arousal system (i.e., monoaminergic neurons, orexinergic neurons) send widespread ascending projections to the cerebral cortex, stimulating cortical desynchronization with high frequency gamma and low frequency theta rhythmic activity. Bottom left, black: MT1 and MT2 receptors expressed in suprachiasmatic neurons, which receive inputs directly from the retinohypothalamic tract (RHT), influenced by light and external stimuli may be likely involved in the switch from wakefulness to NREM sleep. The transition from NREM and REM is controlled by the ventrolateral periaqueductal gray area (vlPAG), containing GABA, glutamate receptors, but also melatonin MT2 receptors. Top right, red: During NREM sleep, two nuclei are particularly active: the reticular thalamus (RT), containing melatonin MT2 and GABA receptors, which is responsible for thalamocortical input to the prefrontal cortex (showing synchronized activity during NREM); and the ventrolateral preoptic area (vlPAG), containing GABA and galanin receptors. They inhibit noradrenergic, serotonergic, cholinergic, histaminergic, and hypocretinergic neurons. These nuclei play a role in the “reciprocal inhibitory” model of the sleep–wake switch. Bottom right, blue: The vlPAG is a putative “REM ON” nucleus, switching the brain to the REM sleep mode. During REM, the sublateral nucleus (SLD), the basal forebrain (BF), and the lateral tegmentum/ pedunculopontine tegmentum (LDT/PPT, rich in acetylcholine receptors) and the ventromedial medulla (VM) neurons are particularly active. The BF is active in REM and wakefulness and inhibited during NREM.
The plethora of studies here reported demonstrating the weak hypnotic properties of exogenous melatonin and the fact that melatonin picks in both nocturnal and diurnal animals at the same time—between 1 and 3 a.m.—(92, 93) leads us to hypothesize that melatonin is not per se a neuromodulator acting on sleep, but rather a pace-maker influencing circadian rhythms among which the circadian regulation of sleep in both diurnal and nocturnal animals. Melatonin likely acts as an “orchestra conductor”: when melatonin peaks (1–3 a.m.) it regulates the expression of MT1, MT2, and other non-melatonin receptors, which are those directly regulating sleep stages. On one hand, the nocturnal overexpression of MT2 receptors in diurnal mammalian increases the propensity to sleep by activating the neurons that trigger NREM sleep (i.e., neurons in the RT). On the other hand, in nocturnal animals, the melatonin peak would down-regulate MT2 receptors while up-regulating MT1 and other receptors involved in wakefulness, for example monoamines (76) and orexin (74) receptors.
In support of this hypothesis, Pinato et al. (94) found that in the diurnal primate Sapajus apella, MT1 and MT2 receptors displayed different reciprocal patterns of expression according to the light/dark cycle in four hypothalamic nuclei, with an apparent inverse expression in the SCN compared with the other three hypothalamic areas. Pinealectomized rats (54) or humans with pineal parenchymal tumors (95) that display significantly altered rhythms in circulating levels of melatonin do not necessarily show sleep impairments, but in contrast, the activation of MT2 receptors or the genetic deletion of either MT1 or MT2 receptors induces significant changes in sleep stages. In line, the non-selective MT1-MT2 agonist tasimelteon, which has been approved for the treatment of non-24-h sleep–wake rhythm disorder in blind people display pharmacological efficacy as a consequence of the resynchronization to a 24-h sleep-wake rhythm (96). Interestingly, this kind of hormonal circadian regulation of the receptors has also been observed for the cortisol peak (occurring early in the morning) and the response of its glucocorticoid and mineralocorticoid receptors (97).
Importantly, similar to cortisol, circulating melatonin may not only play a role in regulating the activity and expression may of its two receptors, but also the expression (98) of clock genes, which in turn regulate a plethora of different cellular functions.
The data reported in this review indicate that the MT2 receptor is mostly involved in sleep, and less in the regulation of circadian rhythms. In contrast, several studies suggest that the MT1 receptor is mostly involved in the circadian regulation of behavior.
Indeed, in-vitro experiments using SCN slides showed that MT1 receptors control the neuronal firing rate and MT2 receptors the phase shift-circadian rhythm of the neuronal firing (52); however, in in-vivo studies, a MLT injection phase shifted the SCN activity onset of WT but not of MT1KO mice and also accelerated the entertainment to a new light-dark cycle of WT but not of MT1KO mice (52, 99), suggesting that MT1 receptor is involved in circadian regulation.
In keeping, MT1KO mice show no light/dark differences in circulating corticosterone levels (76), and unlike WT and MT2KO mice, no light/dark differences in the duration of REM sleep (53). Finally, the abundance of MT1 compared with MT2 receptors in the SCN (65) may also suggest a prime implication of MT1 receptor in circadian regulation.
Further research is necessary to validate this hypothesis linking melatonin, melatonin receptors, circadian rhythms and sleep. Within this context, it will be important to investigate the pathophysiological role of the recently characterized MT1/MT2 heteromers (17), but also of possible heterooligomers between melatonin receptors and 5-HT2c receptors. Notably, 5-HT2c receptors are present in considerable amounts at the level of the SCN (100) and their activation also modulate clock gene expression (101).
Melatonin is an important modulator of the sleep/wake cycle by activating MT1 and MT2 receptors, even if some authors have also hypothesized that melatonin can have MT1/MT2 receptor-independent hypnotic effects (102). Using different experimental approaches, melatonin receptors have been shown to be present in many brain areas/nuclei implicated in the control of the sleep/wake cycle. Importantly, the most recent studies indicate that the two receptor subtypes are differently expressed in regions involved in REM or NREM sleep. For example, the MT2 is uniquely located in the reticular thalamus, an area involved in NREM triggering. In contrast, the MT1 receptor is found in the PFH, involved in REM, as well as in the dorsal raphe nucleus and the locus coeruleus, which are either active, slightly active, or silent according to the wakefulness, NREM, and REM sleep stages, respectively. The neural circuits implicated in the regulation of the sleep/wake cycle have yet to be completely elucidated, and may represent an interesting target for the application of the novel technologies of optogenetics and genetic manipulation which would allow for the activation or inactivation of single receptors in specific areas. The current knowledge we have summarized here suggests that the two melatonin receptors subtypes can have either complementary or opposing effects in NREM and REM sleep, likely because of their different expression in brain areas differently implicated in the regulation of the sleep/wake cycle. These findings result mainly result from preclinical studies genetically and/or pharmacologically targeting MT1 or MT2 receptors, and partially explain the limited efficacy as hypnotics of melatonin or non-selective MT1/MT2 receptor agonists in clinical studies. While the possible role of MT2 receptor in modulating sleep stages has been confirmed by studies in MT2 receptor knockout mice and with compounds activating selectively the MT2 receptor subtype, research on MT1 receptors is still limited to findings in MT1 receptor knockout mice. The development of selective ligands for the MT1 receptor subtype will allow us to test their effects upon the sleep/wake cycle, thus increasing our understanding of the neurobiological role of both MT1 and MT2 receptors in sleep.
Most preclinical research investigating the potential hypnotic effects of selective MT2 agonists/partial agonists has been conducted following only one or a few injections of the drug. No studies have evaluated the effects of a chronic treatment with these different melatonergic compounds on the sleep/wake cycle. This is particularly noteworthy since hypnotics are often prescribed in humans for long periods.
Another important issue arising from the reviewed literature is the importance of considering the time of administration of melatonergic compounds. Comparing preclinical and clinical studies, in humans the treatment has been done early or late (before going to sleep) during the day, and in animals during the light (inactive) or dark (active) phase of the day. Given the circadian variations in the endogenous levels of melatonin and likely in the expression of the two melatonin receptors, it is not surprising that different and/or apparently contrasting findings have been described. Therefore, chronopharmacology should become a leitmotif when discussing the potential implications of the novel findings linking the melatonin system to sleep but also to wider biological/pharmacological issues.
The history of pharmacology indeed has taught us that receptor-selective ligands are superior to the respective neurotransmitter itself. For example, serotonin or the precursor tryptophan is less effective than SSRIs for depression or 5-HT2A antagonists for psychosis. Similarly, selective MT1 or MT2 ligands may be therapeutically more effective than melatonin in the treatment of sleep disorders.
In conclusions, given the lack of medications specifically registered for treating either NREM or REM sleep disorders and the fact that MT1 and MT2 receptors seem to modulate the two sleep stages differently, the future development of selective MT1 or MT2 receptor ligands may help to answer this medical need that afflicts a considerable percentage of the population in industrialized countries.
GG and SC conceived the study, collected data, and wrote the review.
GG is an inventor and assignee of patents for selective melatonin ligands.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
GG received grants from the Canadian Institute of Health Research (CIHR), Fond for health research, Quebec (FRQS), Consortium for Drug Discovery, Quebec (CQDM), and the Ministry of Economy, Science and Innovation of Quebec (MESI) for research on melatonin receptors and sleep. SC is supported in part by a 2017 NARSAD Young Investigator Grant from the Brain and Behavior Research Foundation. We thank Justine Enn for help on editing the manuscript, and Danilo De Gregorio and Martina Dick for help in preparing Figure 1.
1. Tononi G, Cirelli C. Sleep and the price of plasticity: from synaptic and cellular homeostasis to memory consolidation and integration. Neuron. (2014) 81:12–34. doi: 10.1016/j.neuron.2013.12.025
2. Atkin T, Comai S, Gobbi G. Drugs for Insomnia beyond Benzodiazepines: pharmacology, clinical applications, and discovery. Pharmacol Rev. (2018) 70:197–245. doi: 10.1124/pr.117.014381
3. Berry RB, Brooks R, Gamaldo CE, Harding SM, Lloyd RM, Marcus CL, Vaughn BV. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications, Version 2.2. American Academy of Sleep Medicine: Darien, IL (2015).
4. Stickgold R. Sleep-dependent memory consolidation. Nature. (2005) 437:1272–8. doi: 10.1038/nature04286
5. Tasali E, Leproul R, Ehrmann DA, Van Cauter E. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci USA. (2008) 105:1044–9. doi: 10.1073/pnas.0706446105
6. Gagnon JF, Postuma RB, Mazza S, Doyon J, Montplaisir J. Rapid-eye-movement sleep behaviour disorder and neurodegenerative diseases. Lancet Neurol. (2006) 5:424–32. doi: 10.1016/S1474-4422(06)70441-0
7. Kahn M, Sheppes G, Sadeh A. Sleep and emotions: bidirectional links and underlying mechanisms. Int Psychophysiol J. (2013) 89:218–28. doi: 10.1016/j.ijpsycho.2013.05.010
8. Thase ME. Depression and sleep: pathophysiology and treatment. Dialogues Clin Neurosci. (2006) 8:217–26.
12. Mayers AG, Baldwin DS. Antidepressants and their effect on sleep. Hum Psychopharmacol. (2005) 20:533–59. doi: 10.1002/hup.726
13. Reite M. Sleep disorders presenting as psychiatric disorders. Psychiatr Clin North Am. (1998) 21:591–607. doi: 10.1016/S0193-953X(05)70025-3
14. Dubocovich ML, Delagrange P, Krause DN, Sugden D, Cardinali DP, Olcese J. International Union of Basic and Clinical Pharmacology. LXXV nomenclature classification, and pharmacology of G protein-coupled melatonin receptors. Pharmacol Rev. (2010) 62:343–80. doi: 10.1124/pr.110.002832
15. Jockers R, Delagrange P, Dubocovich ML, Markus RP, Renault N, Tosini G, et al. Update on melatonin receptors: IUPHAR review 20. Br Pharmacol J. (2016) 173:2702–25. doi: 10.1111/bph.13536
16. Oishi A, Cecon E, Jockers R. Melatonin receptor signaling: impact of receptor oligomerization on receptor function. Int Rev Cell Mol Biol. (2018) 338:59–77. doi: 10.1016/bs.ircmb.2018.02.002
17. Kamal M, Gbahou F, Guillaume JL, Daulat AM, Benleulmi-Chaachoua A, Luka M, et al. Convergence of melatonin and 5-HT signaling at MT2/5-HT2C receptor heteromers. J Biol Chem. (2015) 290:11537–46. doi: 10.1074/jbc.M114.559542
18. Brzezinski A, Vangel MG, Wurtman RJ, Norrie G, Zhdanova I, Ben-Shushan A, et al. Effects of exogenous melatonin on sleep: a meta-analysis. Sleep Med Rev. (2005) 9:41–50. doi: 10.1016/j.smrv.2004.06.004
19. Buscemi N, Vandermeer B, Hooton N, Pandya R, Tjosvold L, Hartling L, et al. Efficacy and safety of exogenous melatonin for secondary sleep disorders and sleep disorders accompanying sleep restriction: meta-analysis. Br Med J. (2006) 332:385–8C. doi: 10.1136/bmj.38731.532766.F6
20. van den Heuvel CJ, Ferguson SA, Macchi MM, Dawson D. Melatonin as a hypnotic: Con. Sleep Med Rev. (2005) 9:71–80. doi: 10.1016/j.smrv.2004.07.001
21. Zhdanova IV. Melatonin as a hypnotic: Pro. Sleep Med Rev. (2005) 9:51–65. doi: 10.1016/j.smrv.2004.04.003
22. Holmes SW, Sugden D. Effects of melatonin on sleep and neurochemistry in the rat. Br. Pharmacol J. (1982) 76:95–101. doi: 10.1111/j.1476-5381.1982.tb09194.x
23. Ochoa-Sanchez R, Comai S, Spadoni G, Bedini A, Tarzia G, Gobbi G. Melatonin, selective and non-selective MT1/MT2 receptors agonists: differential effects on the 24-h vigilance states. Neurosci Lett. (2014) 561:156–61. doi: 10.1016/j.neulet.2013.12.069
24. Wang F, Li J, Wu C, Yang J, Xu F, Zhao Q. The GABA(A) receptor mediates the hypnotic activity of melatonin in rats. Pharmacol Biochem Behav. (2003) 74:573–8. doi: 10.1016/S0091-3057(02)01045-6
25. Mailliet F, Galloux P, Poisson D. Comparative effects of melatonin, zolpidem and diazepam on sleep, body temperature, blood pressure and heart rate measured by radiotelemetry in Wistar rats. Psychopharmacology. (2001) 156:417–26. doi: 10.1007/s002130100769
26. Mendelson WB, Gillin JC, Dawson SD, Lewy AJ, Wyatt RJ. Effects of melatonin and propranolol on sleep of the rat. Brain Res. (1980) 201:240–4. doi: 10.1016/0006-8993(80)90793-3
27. Mouret J, Coindet J, Chouvet G. Effect of pinealectomy on sleep stages and rhythms of male rat. Brain Res. (1974) 81:97–105. doi: 10.1016/0006-8993(74)90480-6
28. Huber R, Deboer T, Schwierin B, Tobler I. Effect of melatonin on sleep and brain temperature in the Djungarian hamster and the rat. Physiol Behav. (1998) 65:77–82. doi: 10.1016/S0031-9384(98)00125-5
29. Miyamoto M, Nishikawa H, Doken Y, Hirai K, Uchikawa O, Ohkawa S. The sleep-promoting action of ramelteon (TAK-375) in freely moving cats. Sleep. (2004) 27:1319–25. doi: 10.1093/sleep/27.7.1319
30. Tobler I, Jaggi K, Borbely AA. Effects of melatonin and the melatonin receptor agonist S-20098 on the vigilance states, eeg spectra, and cortical temperature in the rat. Pineal Res J. (1994) 16:26–32. doi: 10.1111/j.1600-079X.1994.tb00078.x
31. Yukuhiro N, Kimura H, Nishikawa H, Ohkawa S, Yoshikubo S, Miyamoto M. Effects of ramelteon (TAK-375) on nocturnal sleep in freely moving monkeys. Brain Res. (2004) 1027:59–66. doi: 10.1016/j.brainres.2004.08.035
32. Descamps A, Rousset C, Millan MJ, Spedding M, Delagrange P, Cespuglio R. Influence of the novel antidepressant and melatonin agonist/serotonin2C receptor antagonist, agomelatine, on the rat sleep-wake cycle architecture. Psychopharmacology. (2009) 205:93–106. doi: 10.1007/s00213-009-1519-2
33. Hardeland R. Tasimelteon, a melatonin agonist for the treatment of insomnia and circadian rhythm sleep disorders. Curr Opin Investig Drugs. (2009) 10:691–701.
34. Ochoa-Sanchez R, Comai S, Lacoste B, Bambico FR, Dominguez-Lopez S, Spadoni G, et al. Promotion of non-rapid eye movement sleep and activation of reticular thalamic neurons by a novel MT2 melatonin receptor ligand. J Neurosci. (2011) 31:18439–52. doi: 10.1523/JNEUROSCI.2676-11.2011
35. Fisher SP, Davidson K, Kulla A, Sugden D. Acute sleep-promoting action of the melatonin agonist, ramelteon, in the rat. Pineal Res J. (2008) 45:125–32. doi: 10.1111/j.1600-079X.2008.00565.x
36. Fisher SP, Sugden D. Sleep-promoting action of IIK7, a selective MT2 melatonin receptor agonist in the rat. Neurosci Lett. (2009) 457:93–6. doi: 10.1016/j.neulet.2009.04.005
37. Mini L, Wang-Weigand S, Zhang J. Effects of ramelteon 8 mg on latency to persistent sleep in adults with severe sleep-initiation difficulty; Post-hoc analysis of a 5-week trial. Sleep. (2007) 30:A243.
38. Mini LJ, Wang-Weigand S, Zhang J. Self-reported efficacy and tolerability of ramelteon 8 mg in older adults experiencing severe sleep-onset difficulty. Am J Geriatr Pharmacother. (2007) 5:177–84. doi: 10.1016/j.amjopharm.2007.09.004
39. Roth T, Stubbs C, Walsh JK. Ramelteon (TAK-375), a selective MT1/MT2-receptor agonist, reduces latency to persistent sleep in a model of transient insomnia related to a novel sleep environment. Sleep. (2005) 28:303–7. doi: 10.1093/sleep/28.3.303
41. Levine LR, Smith BP. LY 156735, a melatonin analog, reduces sleep-onset latency in patients with moderate sleep-onset insomnia. In: 37th Annual Meeting of American College of Neuropsychopharmacology. Las Croabas (1998).
42. Zemlan FP, Mulchahey JJ, Scharf MB, Mayleben DW, Rosenberg R, Lankford A. The efficacy and safety of the melatonin agonist beta-methyl-6-chloromelatonin in primary insomnia: A randomized, placebo-controlled, crossover clinical trial. J Clin Psychiatry. (2005) 66:384–90. doi: 10.4088/JCP.v66n0316
43. Roth T, Seiden D, Sainati S, Wang-Weigand S, Zhang J, Zee P. Effects of ramelteon on patient-reported sleep latency in older adults with chronic insomnia. Sleep Med. (2006) 7:312–8. doi: 10.1016/j.sleep.2006.01.003
44. Rajaratnam SM, Polymeropoulos MH, Fisher DM, Roth T, Scott C, Birznieks G, et al. Melatonin agonist tasimelteon (VEC-162) for transient insomnia after sleep-time shift: two randomised controlled multicentre trials. Lancet. (2009) 373:482–91. doi: 10.1016/S0140-6736(08)61812-7
45. Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet. (2018) 391:1357–66. doi: 10.1016/S0140-6736(17)32802-7
46. Quera Salva MA, Vanier B, Laredo J, Hartley S, Chapotot F, Moulin C, et al. Major depressive disorder, sleep EEG and agomelatine: an open-label study. Int Neuropsychopharmacol J. (2007) 10:691–6. doi: 10.1017/S1461145707007754
47. Lemoine P, Guilleminault C, Alvarez E. Improvement in subjective sleep in major depressive disorder with a novel antidepressant, agomelatine: randomized, double-blind comparison with venlafaxine. J Clin Psychiatry. (2007) 68:1723–32. doi: 10.4088/JCP.v68n1112
48. Laudon M, Katz A, Metzger D, Staner L, Pross N, Cornette F, et al. Tolerability, pharmacokinetic and pharmacodynamic evaluation of multiple ascending doses of Neu-P11 in insomnia patients. Sleep. (2012). 35:A221.
49. Liu Y-Y, Yin D, Chen L, Qu W-M, Chen C-R, Laudon M, et al. Piromelatine exerts antinociceptive effect via melatonin, opioid, and 5HT1A receptors and hypnotic effect via melatonin receptors in a mouse model of neuropathic pain. Psychopharmacology. (2014) 231:3973–85. doi: 10.1007/s00213-014-3530-5
50. Neurim Pharmaceuticals. Neurim Pharmaceuticals Announces Positive Phase 2 Clinical Trial Results of Piromelatine for the Treatment of Insomnia [Press Release]. (2013). Available online at: http://www.neurim.com/news/2013-02-18/positive-phase-2-clinical-trial-results-of-piromelatine-for-the-treatment-of-insomnia/
51. Pace-Schott EF, Hobson JA. The neurobiology of sleep: genetics, cellular physiology and subcortical networks. Nat Rev Neurosci. (2002) 3:591–605. doi: 10.1038/nrn895
52. Dubocovich ML. Melatonin receptors: role on sleep and circadian rhythm regulation. Sleep Med. (2007) 8:34–42. doi: 10.1016/j.sleep.2007.10.007
53. Comai S, Ochoa-Sanchez R, Gobbi G. Sleep-wake characterization of double MT(1)/MT(2) receptor knockout mice and comparison with MT(1) and MT(2) receptor knockout mice. Behav Brain Res. (2013) 243:231–8. doi: 10.1016/j.bbr.2013.01.008
54. Fisher SP, Sugden D. Endogenous melatonin is not obligatory for the regulation of the rat sleep-wake cycle. Sleep. (2010) 33:833–40. doi: 10.1093/sleep/33.6.833
55. Niranjan R, Nath C, Shukla R. Melatonin attenuated mediators of neuroinflammation and alpha-7 nicotinic acetylcholine receptor mRNA expression in lipopolysaccharide (LPS) stimulated rat astrocytoma cells, C6. Free Radic Res. (2012) 46:1167–77. doi: 10.3109/10715762.2012.697626
56. Parada E, Buendia I, Leon R, Negredo P, Romero A, Cuadrado A, et al. Neuroprotective effect of melatonin against ischemia is partially mediated by alpha-7 nicotinic receptor modulation and HO-1 overexpression. Pineal Res J. (2014) 56:204–12. doi: 10.1111/jpi.12113
57. Chuang JI, Chen SS, Lin MT. Melatonin decreases brain serotonin release, arterial pressure and heart rate in rats. Pharmacology. (1993) 47:91–7. doi: 10.1159/000139083
58. Monnet FP. Melatonin modulates [3h]serotonin release in the rat hippocampus: effects of circadian rhythm. Neuroendocrinol J. (2002) 14:194–9. doi: 10.1046/j.0007-1331.2001.00761.x
59. Lopez-Canul M, Comai S, Dominguez-Lopez S, Granados-Soto V, Gobbi G. Antinociceptive properties of selective MT2 melatonin receptor partial agonists. Eur. Pharmacol J. (2015) 764:424–32. doi: 10.1016/j.ejphar.2015.07.010
60. Lopez-Canul M, Palazzo E, Dominguez-Lopez S, Luongo L, Lacoste B, Comai S, et al. Selective melatonin MT2 receptor ligands relieve neuropathic pain through modulation of brainstem descending antinociceptive pathways. Pain. (2015) 156:305–17. doi: 10.1097/01.j.pain.0000460311.71572.5f
61. Comai S, Posa L, Ochoa-Sanchez R, Spadoni G, Gobbi GJEN. Neuropsychopharmacological properties of novel melatonin MT1 receptor ligands. Eur. Neuropsychopharmacol. (2017) 27:S569. doi: 10.1016/S0924-977X(17)31098-2
63. Dubocovich ML, Yun K, Al-Ghoul WM, Benloucif S, Masana MI. Selective MT2 melatonin receptor antagonists block melatonin-mediated phase advances of circadian rhythms. FASEB J. (1998) 12:1211–20. doi: 10.1096/fasebj.12.12.1211
64. McShane BB, Galante RJ, Jensen ST, Naidoo N, Pack AI, Wyner A. Characterization of the bout durations of sleep and wakefulness. J Neurosci Methods. (2010) 193:321–33. doi: 10.1016/j.jneumeth.2010.08.024
65. Lacoste B, Angeloni D, Dominguez-Lopez S, Calderoni S, Mauro A, Fraschini F, et al. Anatomical and cellular localization of melatonin MT1 and MT2 receptors in the adult rat brain. Pineal Res J. (2015) 58:397–417. doi: 10.1111/jpi.12224
66. Sugden D, McArthur AJ, Ajpru S, Duniec K, Piggins HD. Expression of mt(1) melatonin receptor subtype mRNA in the entrained rat suprachiasmatic nucleus: a quantitative RT-PCR study across the diurnal cycle. Brain Res Mol Brain Res. (1999) 72:176–82. doi: 10.1016/S0169-328X(99)00222-3
67. Waly N, Hallworth R. Circadian pattern of melatonin MT1 and MT2 receptor localization in the rat suprachiasmatic nucleus. J Circad Rhyth. (2015) 13:1. doi: 10.5334/jcr.ab
68. Liu C, Weaver DR, Jin XW, Shearman LP, Pieschl RL, Gribkoff VK, et al. Molecular dissection of two distinct actions of melatonin on the suprachiasmatic circadian clock. Neuron. (1997) 19:91–102.
69. Steriade M. Cellular substrates of brain rhythms. In: Niedermeyer E, and Lopes Da Silva FH, editors. Electroencephalography: Basic Principles, Clinical Applications, and Related Fields. Philadelphia, PA: Lippincott Williams and Wilkins (1993). p. 31–83.
70. Steriade M. Coherent oscillations and short-term plasticity in corticothalamic networks. Trends Neurosci. (1999) 22:337–45. doi: 10.1016/S0166-2236(99)01407-1
71. Steriade M. Sleep, epilepsy and thalamic reticular inhibitory neurons. Trends Neurosci. (2005) 28:317–24. doi: 10.1016/j.tins.2005.03.007
72. Nunez A, Dossi RC, Contreras D, Steriade M. Intracellular evidence for incompatibility between spindle and delta-oscillations in thalamocortical neurons of cat. Neuroscience. (1992) 48:75–85. doi: 10.1016/0306-4522(92)90339-4
73. Steriade M, Dossi RC, Nunez A. Network modulation of a slow intrinsic oscillation of cat thalamocortical neurons implicated in sleep delta-waves - cortically induced synchronization and brain-stem cholinergic suppression. Neurosci J. (1991) 11:3200–17. doi: 10.1523/JNEUROSCI.11-10-03200.1991
74. Sharma R, Sahota P, Thakkar MM. Melatonin promotes sleep in mice by inhibiting orexin neurons in the perifornical lateral hypothalamus. Pineal Res J. (2018) 65:e12498. doi: 10.1111/jpi.12498
75. Gobbi G, Comai S. Sleep well. Untangling the role of melatonin MT1 and MT2 receptors in sleep. Pineal Res J. (2018) 26:e12544. doi: 10.1111/jpi.12544
76. Comai S, Ochoa-Sanchez R, Dominguez-Lopez S, Bambico FR, Gobbi G. Melancholic-Like behaviors and circadian neurobiological abnormalities in melatonin MT1 receptor knockout mice. Int Neuropsychopharmacol J. (2015) 18:pyu075. doi: 10.1093/ijnp/pyu075
77. Scammell TE, Arrigoni E, Lipton JO. Neural circuitry of wakefulness and sleep. Neuron. (2017) 93:747–65. doi: 10.1016/j.neuron.2017.01.014
78. Baba K, Benleulmi-Chaachoua A, Journe AS, Kamal M, Guillaume JL, Dussaud S, et al. Heteromeric MT1/MT2 melatonin receptors modulate photoreceptor function. Sci Signal. (2013) 6:ra89. doi: 10.1126/scisignal.2004302
79. Borjigin J, Zhang LS, Calinescu AA. Circadian regulation of pineal gland rhythmicity. Mol Cell Endocrinol. (2012) 349:13–9. doi: 10.1016/j.mce.2011.07.009
80. Kalsbeek A, Buijs RM. Output pathways of the mammalian suprachiasmatic nucleus: coding circadian time by transmitter selection and specific targeting. Cell Tissue Res. (2002) 309:109–18. doi: 10.1007/s00441-002-0577-0
81. Witt-Enderby PA, Bennett J, Jarzynka MJ, Firestine S, Melan MA. Melatonin receptors and their regulation: biochemical and structural mechanisms. Life Sci. (2003) 72:2183–98. doi: 10.1016/S0024-3205(03)00098-5
82. Chou TC, Bjorkum AA, Gaus SE, Lu J, Scammell TE, Saper CB. Afferents to the ventrolateral preoptic nucleus. J. Neurosci. (2002) 22:977–90. doi: 10.1523/JNEUROSCI.22-03-00977.2002
83. Sakai K. Sleep-waking discharge profiles of median preoptic and surrounding neurons in mice. Neuroscience. (2011) 182:144–61. doi: 10.1016/j.neuroscience.2011.03.010
84. Adamantidis A, Carter MC, de Lecea L. Optogenetic deconstruction of sleep-wake circuitry in the brain. Front. Mol. Neurosci. (2010) 2:31. doi: 10.3389/neuro.02.031.2009
85. Portaluppi F, Cortelli P, Avoni P, Vergnani L, Maltoni P, Pavani A, et al. Progressive disruption of the circadian rhythm of melatonin in fatal familial insomnia. J Clin Endocrinol Metab. (1994) 78:1075–8.
86. Steriade M, Timofeev I. Neuronal plasticity in thalamocortical networks during sleep and waking oscillations. Neuron. (2003) 37:563–76. doi: 10.1016/S0896-6273(03)00065-5
87. Purves D, Augustine GJ, Fitzpatrick D, Hall WC, Lamantia A.-S, McNamara JO, et al. Neuroscience. Sunderland, MA: Sinauer Associates Inc (2004).
88. Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. (2001) 24:726–31. doi: 10.1016/S0166-2236(00)02002-6
89. Aston-Jones G, Bloom FE. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. Neurosci J. (1981) 1:876–86. doi: 10.1523/JNEUROSCI.01-08-00876.1981
90. Page ME, Valentino RJ. Locus coeruleus activation by physiological challenges. Brain Res Bull. (1994) 35:557–60. doi: 10.1016/0361-9230(94)90169-4
91. Florin-Lechner SM, Druhan JP, Aston-Jones G, Valentino RJ. Enhanced norepinephrine release in prefrontal cortex with burst stimulation of the locus coeruleus. Brain Res. (1996) 742:89–97. doi: 10.1016/S0006-8993(96)00967-5
92. Karasek M. Does melatonin play a role in aging processes? J Physiol Pharmacol. (2007) 58(Suppl. 6):105–13.
93. Reiter RJ, Craft CM, Johnson JE Jr, King TS, Richardson BA, Vaughan GM, et al. Age-associated reduction in nocturnal pineal melatonin levels in female rats. Endocrinology. (1981) 109:1295–7. doi: 10.1210/endo-109-4-1295
94. Pinato L, Ramos D, Hataka A, Rossignoli PS, Granado MDJ, Mazzetto MC Day/night expression of MT1 and MT2 receptors in hypothalamic nuclei of the primate Sapajus apella. J Chem Neuroanat. (2017) 81:10–7. doi: 10.1016/j.jchemneu.2017.01.005
95. Leston J, Mottolese C, Champier J, Jouvet A, Brun J, Sindou M, et al. Contribution of the daily melatonin profile to diagnosis of tumors of the pineal region. Neurooncol J. (2009) 93:387–94. doi: 10.1007/s11060-008-9792-1
96. Neubauer DN. Tasimelteon for the treatment of non-24-hour sleep-wake disorder. Drugs Today. (2015) 51:29–35. doi: 10.1358/dot.2015.51.1.2258364
97. Chung S, Son GH, Kim K. Circadian rhythm of adrenal glucocorticoid: Its regulation and clinical implications. Biochim Biophys Acta. (2011) 1812:581–91. doi: 10.1016/j.bbadis.2011.02.003
98. James FO, Cermakian N, Boivin DB. Circadian rhythms of melatonin, cortisol, and clock gene expression during simulated night shift work. Sleep. (2007) 30:1427–36. doi: 10.1093/sleep/30.11.1427
99. Jin X, von Gall C, Pieschl RL, Gribkoff VK, Stehle JH, Reppert SM, et al. Targeted disruption of the mouse Mel(1b) melatonin receptor. Mol Cell Biol. (2003) 23:1054–60. doi: 10.1128/MCB.23.3.1054-1060.2003
100. Moyer RW, Kennaway DJ. Immunohistochemical localization of serotonin receptors in the rat suprachiasmatic nucleus. Neurosci Lett. (1999) 271:147–50. doi: 10.1016/S0304-3940(99)00536-4
101. Varcoe TJ, Kennaway DJ. Activation of 5-HT2C receptors acutely induces Per1 gene expression in the rat SCN in vitro. Brain Res. (2008) 1209:19–28. doi: 10.1016/j.brainres.2008.02.091
Keywords: melatonin, MT1 receptor, MT2 receptor, sleep, REM, NREM
Citation: Gobbi G and Comai S (2019) Differential Function of Melatonin MT1 and MT2 Receptors in REM and NREM Sleep. Front. Endocrinol. 10:87. doi: 10.3389/fendo.2019.00087
Received: 16 November 2018; Accepted: 31 January 2019;
Published: 01 March 2019.
Edited by:
Ralf Jockers, Université Paris-Sorbonne, FranceReviewed by:
Gianluca Tosini, Morehouse School of Medicine, United StatesCopyright © 2019 Gobbi and Comai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gabriella Gobbi, Z2FicmllbGxhLmdvYmJpQG1jZ2lsbC5jYQ==
Stefano Comai, Y29tYWkuc3RlZmFub0Boc3IuaXQ=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.